Most of us are fairly comfortable with setting limits for swab samples. However, I still see some confusion in setting limits for rinse samples. This Cleaning Memo clarifies what is involved in rinse sampling and how limits could be appropriately set. It also includes examples of how not to set rinse limits (or at least how not to set them unless you want the limits to be lower than what is required or unless you want to set limits inappropriately high).
The first thing to be emphasized is that limits are to be set based on the possibility of residues on cleaned equipment surfaces being transferred to the next product manufactured in that equipment. So when we set swab limits, we determine the acceptable level for the residue in terms of mass per unit area, such as mcg/cm2, what I typically call a “L3” limit. There is nothing new here. But the important thing to remember if we are doing rinse sampling is that the allowable amount on the equipment surfaces does not change. We should use the same L3 value for limits for both rinse sampling and swab sampling.
Now we get to the critical part. If we pass fixed volume of rinse water (I will use water in my explanation, but if an organic solvent or anther liquid applies for rinse sampling, you can easily make the substitution as applicable for that situation), and if the water quantitatively removes the residue from the surface, then it is possible to collect the entire rinse volume, measure the residue level (concentration) in the rinse water, and determine how much was present on the equipment surfaces. That calculation is done by multiplying the concentration of residue by the total “rinse volume” (a term to be discussed later) to give an M2 value (see the Cleaning Memo of June 2022 for a discussion of “M” values). The M2 value is then divided by the surface area sampled to give an M3 value that can be compared to the calculated L3 value. Sounds simple enough, but there are some possible pitfalls.
Before we get to the pitfalls, here is a simple example of one correct application of this type of limit calculation. In this situation, we first complete the process rinse. Once the cleaning SOP is complete, it is possible to move on to the sampling rinse, which is what I typically call a separate sampling rinse (or SSR) that is distinct from the process rinse. Let’s say I pass 50 liters of sampling rinse water through a cleaned 1,000-liter storage tank using a spray ball in a CIP system. If the previously calculated L3 limit were X mcg/cm2 with a surface area of the storage tank of 3,000,000 cm2, then with a measured amount of residue in the rinse water of 12 mcg/mL, the concentration of residue (per surface area ) would be:
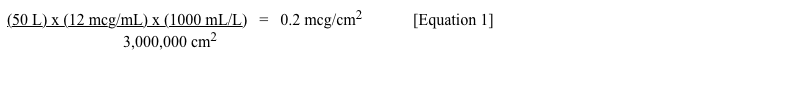
I could then compare that measured result (0.2 mcg/cm2 in the example) to my calculated limit (X mcg/cm2) to determine the acceptability of the cleaning process as measured by a separate sampling rinse. In this situation, I should NOT take my rinse sample from the final portion of the CIP sampling rinse. I should collect the entire 50 L of rinse water, mix it until it is uniform, and take a smaller sample for analysis. If I were to take my sample to be analyzed only from the last portion of the CIP rinse, my measured value would likely be much lower. Why? Because a much larger amount of residue removed from the equipment would likely be present in the first amount of the SSR as it exits from the system. The collected sample from the final portion would likely have a much lower concentration, but what I want is an M4c close to an “average” value.
Now you might be wondering, “How do I do swab sampling now after I have completed the SSR?” Well, you don’t (or if you do, the results are not meaningful). Swab sampling should only be done after the completion of the process rinse; if you try swab sampling after the SRR, theoretically all (or a majority) of the residue from the selected worst-case swab locations may already have been removed by the SSR. Next month we’ll cover the situation of doing both swabbing and rinse sampling in one protocol. So now we’ll move on to the three situations of setting limits if we take a sample from the process rinse.
Okay, now for those who want to take a sample of the process rinse for analysis for residues. In that situation, it is not appropriate to take the entire process rinse, mix until uniform, and then take a subsample to send for analysis. The reason that the measured value in the rinse sample will be extremely high is that it would clearly overstate how much residue is on the surface after completion of the process rinse. Doing so considers residues in the early portions of the process rinse, which is not applicable for what could be left on the equipment surfaces after completion of the cleaning process. Furthermore, if the entire process rinse volume was used for the L4c limit calculation, the residue limit will be driven much lower than scientifically required. So the end result is an unnecessarily low L4c combined with an unnecessarily high M4c, which would in most cases result in a failed protocol.
One way to improve this with a process rinse is to use a series of individual rinses as part of the process rinsing step. For example, rather than rinsing the equipment with 300 L of water in one step, I could do a first rinse with 100 L of water and allow the equipment to drain. I follow with a second rinse of 100 L and allow draining. Then I conclude the process rinse by using 100 L of water as a final portion of the process rinse. I am still using 300 L of water for rinsing, but I am doing it in three steps. So I could conceivably only collect the final 100 L of water, mix it until it is uniform, and send it for analysis. Not only would my rinse water L4c limit be higher by a factor of three (as compared to the situation with using a full 300 L as the “rinse volume” in the calculation for one continuous rinse), but it is likely that my M4c value would be considerably lower because of the better efficiency of rinsing with the three discrete rinses.
A further enhancement is possible if the process rinse is a CIP-type process rinse. In that type of rinse, the final rinse is typically “once through to drain” rather than a recirculating rinse. In this situation, I do not take into consideration the entire volume of the last process rinse. For the sample to be sent for analytical testing, I collect a sample (typically only an adequate amount required for the analysis) from the final portion of that last process rinse. The concentration in that smaller sample should be representative of (or should correlate to) what could be left on equipment after completion of the process rinse.
Now we get to the crucial issue for Case IV– how the limit is determined. As we discussed earlier, the L3 limit does NOT change based on the sampling method. The key is what value we use for the “rinse volume”. What could be used for the rinse volume (in the denominator in an L4c limit) is not the entire last process rinse, nor is it the small volume I collected to send for analysis. Rather, the “rinse volume” amount in the L4c calculation could be an amount of water that would be required to contact all surfaces of the equipment so that all surfaces are “sampled”. In a CIP-type rinse, that volume could be the amount of water used in a riboflavin coverage study, or it could be a reasonable amount based on professional judgment, such as 5% of the volume of the equipment.
Remember that in a CIP application, the water is sprayed into the equipment and does not fill the equipment, but all equipment surfaces are covered. So, for a 1000 L vessel, it might be that 50 L of water would be adequate for coverage; therefore, “sampling” all the surfaces in the CIP application. So in contrast to the Case III example, rather than using 100 L as the rinse volume, I could use 50 L as the rinse volume in the L4c calculation for Case IV.
Note that this approach is applicable for CIP type cleaning regardless of whether the process rinse is 300 L in a continuous manner or a set of three 100 L rinses in sequence. While the L4c limit calculation would be exactly the same, the use of three discrete rinses (each with 100 L) is still preferable because the M4c values are likely to be lower. However, the emphasis in this Cleaning Memo is setting limits, and not on designing the cleaning process so that residues left on the equipment are at lower levels.
Some of you may not have followed this logic in these cases because the way you set L4c rinse limits is by dividing the L2 limit by the “rinse volume”. I have discussed this in training webinars, but not in a Cleaning Memo. So here is the explanation of the care needed in calculating rinse limits in this manner. Using the formula L2/(rinse volume) works as a valid carryover limit calculation only if one is considering rinsing of the entire equipment train as one step (that is, as one process). Here’s why. The formula L2/(rinse volume) is actually derived from the equation below:

This equation becomes simply L2/(rinse volume) there if the two surface areas used are exactly the same value. However, in most cases (at least for most cases in drug product batch manufacture), rinsing is done separately for each equipment item in a train. Therefore, using the equation above is technically incorrect in those situations (because the rinse limits will be set inappropriately high). Also, just for clarification, the usual presentation I use is to base the L4c on L3, with the equation below:
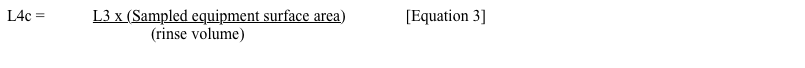
The two presentations (Equation 2 and Equation 3) will give the same L4c limit because L2 divided by “Equipment train surface area” equals L3.
For an earlier presentation of the concept of making an “estimate” of the final portion of the CIP rinse for limits (what I have called “grab sample limits”), please see the Cleaning Memo of January 2009 (as well as an earlier version in October 2005).
For those struggling with understanding these concepts in this current Cleaning Memo, I also suggest that a review be made of the Cleaning Memo of November 2019, entitled “Issues in Rinsing – Part I”.
Next month we will consider issues if we want to do both swab sampling and rinse sampling in one protocol.
Copyright © 2023 by Cleaning Validation Technologies