This is a continuation of the March 2016 Cleaning Memo. If you haven’t read that one (Part1), it would be prudent to do so. In that previous Cleaning Memo we discussed a situation where there were two API synthesis routes for two final APIs, and these were made on the same equipment train comprised of four equipment items. We first looked at setting limits for cleaning after the very last intermediate step. Now we will take a look a slight variation, where we take a look at setting limits for an intermediate in the upstream part of the synthesis chain. We’ll use the same “schematic” to present this situation.
The API’s are API-A and API-B. There are four synthesis steps in the manufacture of each API, each with equipment dedicated to that step. In this example, the equipment for each step is all the equipment associated with that step. Here is a way to view the process, with intermediates Int-A1, Int-A2 and Int-A3 for the manufacture of API-A, and a similar designation for intermediates for the manufacture of API-B.
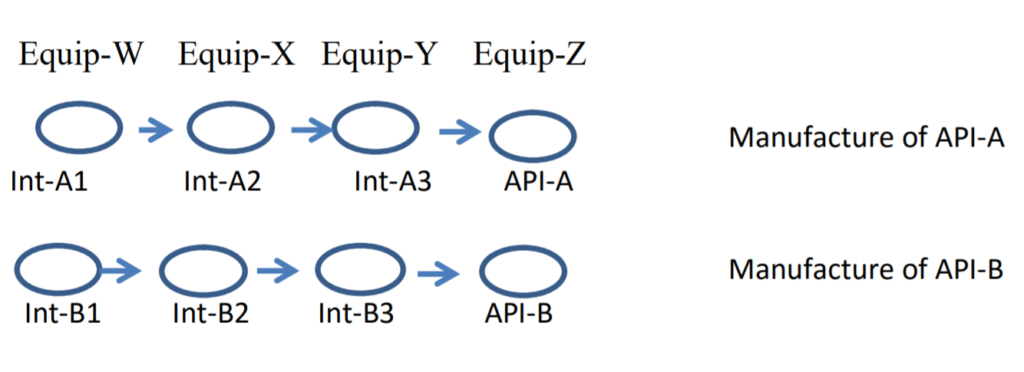
For this new example, we will consider the cleaning of Equip-W after manufacture of Int-A1. Following cleaning, residues from Int-A1 could be left in Equip-W. If the next batch made in Equip-W is another batch of Int-A1, then the situation is just like that of “dedicated” equipment discussed last month (although we will consider another alternative shortly).
Where it gets interesting is if the cleaned Equip-W is used to manufacture a batch of Int-B1 (the first processing step for the manufacture of API-B). In this there are two possibilities. Residues in that cleaned Equip-W, unless cleared by the API-B synthesis process, could transfer down the line and end up in the final API-B. Therefore, limits should be set based on the residues of Int-A1 (those residues left after the cleaning of processing of Int-A1 in Equip-W) transferring to API-B.
But, here’s a second consideration. Let’s assume we manufacture Int-A1 in Equip-W, clean the Equip-W, and then manufacture Int-B1. Then the next step is the processing of Int-B2 in Equip-X, where we follow processing with cleaning. And then the next step is to manufacture Int-A2 in Equip-X. What this might mean is that any Residues of Int-A1 transferred to Int-B1 and then left behind after cleaning of Int-B2 in Equip-X could now be transferred down the line to end up in API-A.
What this means practically is that for cleaning of Int-A1 in Equip-W, I should consider limits if residues of Int-A1 ends up in either API-A or API-B, and the actual validation protocol limit should be set on the lower of the two values (thus negating my statement several paragraphs previously about the possibility of treating it like “dedicated” equipment”). Using Equations I, II and III from last month’s Cleaning Memo, the approach should be to calculate a L3 value for Int- A1 in API-A and an L3 value for Int-A1 in API-B, and then use the lower of the two values for a cleaning validation protocol. It should be clear the main difference between the situation presented in this Part 2 and that given in Part 1 is that I must consider the possibility of an intermediate ending up in the final API for both process streams.
It is clearly possible to expand this approach to a larger number of APIs. It is also possible to expand this to a larger number of processing steps. Furthermore, it can be expanded to situation where the same equipment is used for different synthesis step (for example, equipment used to manufacture both Int-A1 and Int-A3, or even Int-A1 and API-A). Whatever the complexity, the principle is exactly the same – how can residues from the any cleaning step end up in a final API.
But remember last month we also mentioned the issue not of health or safety related issues in setting limits, but also the issue of product quality, purity and/or process efficiency. If I do have a situation like the last mentioned in the previous paragraph (both Int-A1 and API-A made in the same equipment), I need to consider the possibility of residues of API-A in the cleaned equipment interfering with the next process step, which might be the processing of Int-A1 or Int-B1. This is something that may or may not have been evaluated as part of the process development for either API-A or API-B.
One question that often comes up is “How do I determine clearance?” What we are trying to determine is to what extent any residue from one synthesis step carries over to the end product made as the next synthesis step in that same equipment. There are least two or three ways.
One is to perform lab studies where for the second synthesis we add at the beginning of that synthesis a fixed (low but measureable) amount of the residue that could be left over from the prior synthesis. For example, I could simulate the synthesis of Int-B1 in the lab, making sure I added a fixed amount of Int-A1 at the beginning of the synthesis. At the end of the synthesis, I measure Int-A1 in the Int-B1. Alternatively, providing Int-B1 is a solid, I could measure Int-A1 in the solvent used for processing, and assume the difference is left in Int-B1. This would tell me the clearance of Int-A1 by the Int-B1 process. Note that I would like the amount of Int-A1 that I use for spiking to preferably be an amount where I could claim (with adequate clearance) at least a one-log reduction, although it would be nice to claim as much as a three-log reduction. To balance this, I don’t want to spike with an unreasonably high amount of residue of Int-A1, such that I would significantly interfere with the synthesis.
A second way to demonstrate clearance is to perform a “paper” exercise based on solubility of the residue in the solvent used for the next manufactured product. I’ll illustrate with an example. Let’s say that I have residue Int-A1 and I want to see if it is cleared by the synthesis step of Int-B1. For this example, let’s assume that I use an organic solvent for that synthesis step (of Int-B1), and that Int-B1 is insoluble in that solvent. That is, I can filter out the Int-B1 and dry it to produce my intermediate. I then need four pieces of information to know whether substantial clearance will occur. The first is the solubility of Int-A1 in the solvent. The second is the amount Int-A1 that was left over after cleaning (I get this from my rinse/reflux sampling, although I could use a worst-case value based on the equipment being visually clean). The third is the amount of solvent used for processing in the synthesis step for Int-B1. And the fourth is the amount of solvent left in the “cake” of Int-B1 after filtration (but before drying).
So, if the solubility of Int-A1 in acetone is more than concentration of actual Int-A1 residue in the acetone used for the synthesis of Int-B1 (a key requirement), then the amount carried over to Int-B1 is the amount of Int-A1 left in the cake of Int-B1 after filtration.
The percent clearance (meaning amount removed compared to the total amount originally present) can be represented mathematically as:
1 – H/ G x 100
Where:
H is the amount of solvent in the next intermediate after filtration (in units such as g)
G is the amount of solvent used for processing (in units such as g)
This sounds simple, but remember that it depends on the actual concentration of the residue of the intermediate in the solvent being less than the solubility of the intermediate in the solvent at the processing temperature. If the actual concentration of the intermediate residue in the solvent is greater than the solubility, then it may be possible to determine the percent clearance by adding an adjustment factor to account for the insoluble amount of that intermediate residue left in the cake after filtration. Note that in either case, the calculation assumes that whatever is left in the wet cake before drying (if drying is done) will be carried over to that intermediate.
The example given is an easy one, and other specific cases may be more complex. Note further that if there are multiple synthesis steps following, I can determine clearance by one or more steps. And, there also may be situations where I just want to show clearance in the final API synthesis step.
A third way to demonstrate clearance is to actually measure residues in production equipment using production processes. That is, after manufacturing Int-A1, I would sample the equipment by rinse or solvent reflux (this would better allow me to quantitate the total amount of residue in the cleaned equipment). Unfortunately, if I do that, I cannot then process Int-B1 in the same equipment, because I would have effectively reduced the residue levels and would probably find excellent clearance if I were to immediately process Int-B1 after the rinse/reflux sampling. No. What I have to do is perform another synthesis of Int-A1, and after cleaning, then manufacture Int-B1. I then measure the total amount of residues of Int-A1 in Int-B1 to allow me to calculate the percent clearance. In this case I am assuming that the residue of Int-A1 left in the equipment after cleaning of each lot is fairly reproducible.
Note in the three cases given, clearance may not just be by a subsequent purification step. The actual synthesis step may be the “purification” steps that provide clearance, even though we might not ordinarily identify that step as a “purification” step.
We still have some more topics to cover, which we’ll cover in next month’s Cleaning Memo. Those include examples of cleaning equipment after manufacture of the final API, as well as consideration of total carryover of all residues into the final API and consideration of “default” values for limits.