In a grouping (matrixing) approach to cleaning validation, one product is selected as the “worst-case”, and cleaning validation is done on that product. Successful validation of that product constitutes coverage of validation for all products in the group. My recommended approach is to select the “most difficult to clean” product in the group as the product to perform my validation protocols on, but to set limits for the active in that product at the lowest limit of any active for products in the group.
But, I often see an approach where a variety of factors are involved in selecting the worst-case product, and that among these factors is one item called “toxicity” (or a similar designation). In this approach, the toxicity may be based on PDE/ADE values, on a 1/1000 dose criterion, or on other toxicity data. While this is a common practice, it is not one that I typically recommend. Why?
Perhaps it is because I generally like to select the product I perform my protocol on as the “most difficult to clean” product. And I am not sure how the toxicity of a product makes it more (or less) difficult to clean (other than it has to be cleaned to a lower limit). Others may select the product to perform protocols on as a “worst case” product, and using that “worst case” criterion (rather than my “most difficult to clean” criterion), I can understand why some may include toxicity as one of the factors in making a product a “worst case”.
But is toxicity a relevant factor to consider in a grouping (matrixing) approach? Of course! Which is why in my recommended approach, I specify performing a protocol on the most difficult to clean product, but setting limits for that product based on the lowest limit of any product in the group.
Here is an example that may illustrate a possible pitfall in worst-case selection that includes toxicity as one of the determining factors for picking the product to use for the protocol runs. Let’s assume that we have two products (A and B) with the following characteristics, and let’s assume we have only two factors in determining the worst case. The factors are solubility of the active and PDE value of the active. Let’s assume that equal weight is given to each factor, and that each is assessed on a scale of one to five.
Here is my example rating system:

Let’s assume the “point” values for Product A and Product B are as follows:
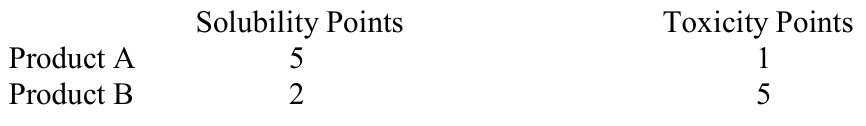
That is, Product A is the least soluble and Product B is the most toxic.
In an approach where I either multiply the points or add the points, Product B is the worst case (most points). In this situation, I would then perform my validation protocol on Product B and set my limit based on the Product B limits. Note in this example that this limit would be used whether I selected either the limit of the worst-case product or the lowest limit among all products in the group. Further note that I am assuming the L3 limit (the limit per surface area) of the active in Product B would be lower than the L3 limit of the active in Product A.
The concern about this approach is “how do I know Product A would effectively be cleaned to its limit if, based on solubility of the active, Product A was the most difficult to clean?” The answer is “I don’t know for sure, even though it might be the case.”
The case presented is meant to be an extreme case for illustration purposes. For most situations, it may well be that including “toxicity” as one criterion in selection of the “worst-case” product may not make a difference.
Furthermore, note that my recommendation of performing the protocol on the “most difficult to clean” product, but using as the limit the lowest limit of any product in the group, would mean for this example evaluating Product A but setting the limit at the limit of Product B. This would assure that Product B, an easier-to-clean product, would be able to be cleaned down to its calculated limit.
Some may object to that recommendation because they would always want to include a highly hazardous product in a protocol (assuming Product B has a highly hazardous active). If that were the case, I would suggest separate validation protocols for Product A and Product B. If there were more products, it might be possible to form two groups, one group of highly hazardous actives and one group with actives that were not highly hazardous.
Finally, note that the scheme presented in the “point system” example is just there for illustration purposes. The point scheme used in a given company should be based on an understanding of products cleaned and an associated risk assessment.