There have been past publications on setting limits for cleaning validation for manufacture of small molecule API’s by an organic synthesis route (including the 2014 APIC guidance and some of my older publications). In my mind, none of them fully address all the complex issues involved in setting limits for these situations, which involve setting limits for cleaning of the final API as well as setting limits for cleaning of intermediates in the synthesis route. It may be a simple matter if equipment is dedicated to the synthesis of one active, and each of the synthesis steps has its own dedicated equipment. However, where there are multiple API’s manufactured on the same equipment, and where some equipment may be used for different synthesis steps (that is, both the final API as well as an intermediate may use the same equipment), life becomes much more complex.
Furthermore, it is recognized (such as in ICH Q7) that residues from earlier cleaning steps in a series of synthesis steps may not be critical (and therefore may not require cleaning validation) provided those residues are “cleared” by any subsequent purification process (much like the idea of viral clearance). Some have suggested using a standard figure like using adjustments assuming 90% clearance for limits of residues of those earlier steps. Note that this depends on the solubility of the residue in any subsequent process solvent, as well as the fate that solvent relative to next product. We only have to look at the Viracept example to see that clearance does not always occur; in that situation, there was no evidence that a mutagenic impurity was cleared in the synthesis process.
What follows is one approach to dealing with these situations on a logical basis. Note that this is just one approach; there may be others that are equally good or even better. I will first illustrate this approach with a simple situation of the manufacture of two API’s in the same equipment train. The API’s are API-A and API-B. There are four synthesis steps in the manufacture of each API, each with equipment dedicated to that step. In this example, the equipment for each step is all the equipment associated with that step. Here is a way to view the process, with intermediates Int-A1, Int-A2 and Int-A3 for the manufacture of API-A, and a similar designation for intermediates for the manufacture of API-B.
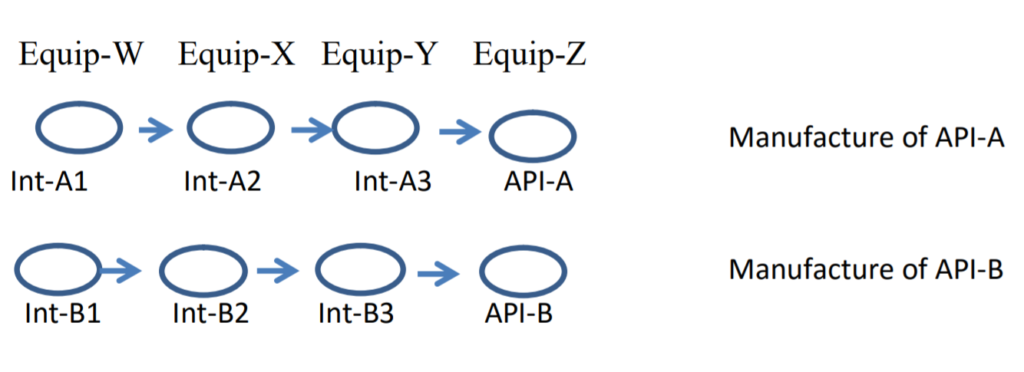
Let’s start with a simple example of cleaning of the last equipment used to produce an intermediate (Equip-Y), where we are dealing with residues of Int-A3 carrying over to the next synthesis in this equipment, which involves a synthesis of a different API, namely Int-B3.
You might ask why I don’t consider the situation where I am cleaning Int-A3 in Equip-Y and the next synthesis in that equipment is to be another batch of Int-A3. The issue here is not unlike dealing with equipment dedicated to one product. The presence of residues of Int-A3 in Equip-Y at the beginning of a subsequent synthesis of Int-A3 in Equip-Y should not affect the amount of carryover of Int-A3 into the next product following cleaning after that subsequent batch. Yes, I may be concerned about the presence of excess amounts of Int-A3 at the beginning of synthesis of the next batch of Int-A3 affecting the synthesis in terms of side reactions or yield. However, this is something that should be evaluated as part of process validation, and ordinarily would not be considered for typical cleaning validation purposes.
Now, back to the example I am focusing on. How is it addressed from a limits perspective when the residue is of Int-A3 left in Equip-Y will potentially affect not API-A, but rather API-B? That is, after cleaning of Equip-Y following synthesis of Int-A3, the next product made in Equip-Y is Int- B3 (and not API-A). Residues transferred to Int-B3 can thus potentially carry over to API-B.
Here is how I would calculate the limit. Note that I am using my shorthand designations of L0, L1, L2, L3 and L4a, L4b and L4c to illustrate this (See my September 2012 Cleaning Memo for more on the use of these designations).
Remember, in this case the limits of Int-A3 is going to be based on what happens if it gets into the API-B synthesis chain and ends up in API-B. You may argue that we should really consider the limit of Int-A3 in Int-B3. While that would be possible, the limit of Int-A3 in Int-B3 will also depend on the parameters of API-B, so it is easier to do this in one calculation rather than a series of two. The L1 (limit of Int-A3 in API-B) can be calculated as:
L1 of Int-A3 in API-B (mg/g) = (L0 of Int-A3) (CF)/(MDD of API-B) Equation I
L2 of Int-A3 in Equip-Y (mg) = (L1) (BS of API-B) Equation II
L3 of Int-A3 in Equip-Y (mg/cm2) = (L2)/(SA of Equip-Y) Equation III
The L4 values (for swab and rinse) can then be calculated just as for typical drug product manufacture (that is, by multiplying L3 by the sampled area and dividing by the sample volume).
Here is a clarification of what other acronyms mean:
MDD is the maximum daily dose (mg) of the final API. It is not the dose of a drug product, but the dose of the API itself.
CF is a new term (at least for me). It is the “clearance factor”, which is a factor to account for clearance of the intermediate as it goes through subsequent process steps (including recrystallization or filtration) that will reduce the level of the species in the final API. This is to be determined for the specific residue and subsequent processing steps. (Note: The APIC guideline assumes a CF of about 5-10; this may or may not apply in your situation. It is important to know the fate of those residues as the residues are carried along in subsequent synthesis steps.) Note that higher clearance factors allow higher limits. The CF that must be considered in this example is not the clearance of Int- A3 residue in the API-A syntheses chain, but rather the clearance in the applicable API-B synthesis chain. The CF value is determined by establishing the percentage of residue remaining in the next product (that is, not removed) through the specified processing steps. As used here, the CF value is 100 divided by that percentage. For example, if there is no clearance and 100% remains in the next product through subsequent processing, then the CF is 100/100, or 1. If clearance is 90%, meaning 10% remains in the next product through, then the CF value is 100/10, or 10. The CF value can be any number greater than or equal to one (≥ 1). If you prefer, you can define a CF based on a value to be used in the denominator of Equation I using similar logic.
BS is the minimum batch size of the final API (in this example, API-B).
SA is the surface area of the equipment used to manufacture the intermediate that was cleaned (in this case, the surface area of Equip-Y). It is not the total shared surface area (as used in drug product manufacture), because an intermediate and an API may not share any equipment.
The purpose of this Cleaning Memo is not to elaborate over how L0 is selected. However, it should be noted that the L0 for an intermediate can be determined as an ADE/PDE value, as an ADI value based on LD50 data, or because of lack of sufficient toxicity data some companies may elect to use the L0 of the final API in the same synthesis chain (based on the intermediate having the same basic structure of the final API).
In the given example, I assumed that only two APIs were made in the series of four equipment items. It should be obvious that if I expanded the number of APIs in the same series of equipment items, I would have to do calculations based on Int-A3 residues getting into API-B, API-C, API-D and so on. I would then use the lowest limit in any of those final APIs for my cleaning validation limit for the cleaning of Int-A3 in Equip-Y. Note that determination of the worst case final API in this situation can be determined based on the lowest BS:MDD ratio of that API only if I assume no clearance (CF = 1) or exactly the same clearance for all cases. The reason is that different CF values for different synthesis routes may significantly change what final API produces the worst case (that is, the lowest L3 limit value). And I must further remember that the clearance of residue of Int-A1 (for example) is not just based on clearance in the API-A manufacturing process chain; I must also consider clearance in any other synthesis chain (or portion of a synthesis chain) where that residue might end up in a final API.
This same approach, with certain significant modifications, can be used for any of the intermediates further upstream in the synthesis chain. We’ll cover that in subsequent Cleaning Memos. We’ll also cover setting limits for the cleaning of the API step itself, as well as address issues relating to final processing of an API where clearance does not occur (such as in a milling step). Finally, we will eventually get to the issue of default limits (that is, limits for L1 or L3 that are used if more stringent than the calculated values) for API manufacture, as well as consideration of total residues in the final API contributed by the cumulative previous cleaning steps.
So stay tuned for more on the subject of limits for small molecule API manufacture in next month’s Cleaning Memo.